Should You Rotate Disinfectants? Industry Experts Weigh In
By Crystal M. Booth, PSC Biotech
 Whether to rotate disinfectants has been debated in the pharmaceutical industry for years, and numerous articles have been written on the subject. It is well understood that disinfectants play an important role in controlling microbial contamination on surfaces. If disinfectants are not used properly, microorganisms may remain on surfaces and proliferate in the environment. There are two schools of thought on the topic of disinfection rotation. This article discusses the concept of disinfectant rotation and the current mainstream industry practice regarding it.
Whether to rotate disinfectants has been debated in the pharmaceutical industry for years, and numerous articles have been written on the subject. It is well understood that disinfectants play an important role in controlling microbial contamination on surfaces. If disinfectants are not used properly, microorganisms may remain on surfaces and proliferate in the environment. There are two schools of thought on the topic of disinfection rotation. This article discusses the concept of disinfectant rotation and the current mainstream industry practice regarding it.
Overview Of Disinfectants And Regulatory Expectations
Validated chemical agents are utilized to combat microbial contamination in facilities. These chemical agents can be classified as sanitizers, disinfectants, or sporicidal agents, based on their efficacy. The general term “disinfectant" is often broadly used to describe these chemical agents. In turn, these validated disinfectants are utilized in cleaning and sanitization programs that are critical elements of an overall contamination control strategy within a facility.1
Many different types of chemicals are available for cleaning, sanitization, or disinfection. The United States Pharmacopeia (USP) <1072> Disinfectants and Antiseptics defines and describes some of the available chemicals. According to USP <1072>, the following properties can impact the effectiveness of the disinfectants being utilized in a facility:2
- pH of a disinfectant2
- intrinsic biocidal activity2
- concentration of the disinfectant2
- contact time2
- It is important to note “contact times” refers to wet contact times. In other words, the material being disinfected must remain wet with the disinfectant for the period of the validated contact time.3
- method of the disinfectant application2 (e.g., spraying, wiping, mopping)4
- nature of the surface disinfected2
- hardness of the water used to dilute the disinfectant2
- temperature of the disinfectant solution or areas to be disinfected2
- amount of organic materials present on the surface being cleaned2
- soil or debris present on the surface2
- type and number of microorganisms present.2
It is up to the end user to decide which disinfectants are right for its cleaning and sanitization program. These decisions are often based on risk assessments, environmental data, industry practice, regulatory expectations, and disinfectant efficacy testing results.
Many regulations and guidance documents discuss the use of disinfectants to destroy microorganisms in the environment. The following list is not intended to be all-inclusive. While these documents provide helpful information on disinfectant programs, not all discuss the principle of disinfectant rotation.
- PDA Technical Report 70: Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities
- PDA Technical Report 13: Fundamentals of an Environmental Monitoring (EM) Program
- USP <1072> Disinfectants and Antiseptics
- U.S. Code of Federal Regulations (CFR) Title 21 Part 211.42 (c)
- U.S. CFR Title 21 Part 211.67 Sec. 211.67
- Guidance for the Industry: Sterile Drug Products Produced by Aseptic Processing —Current Good Manufacturing Practices (GMPs), FDA
- Annex 1: EudraLex, The Rules Governing Medicinal Products in the European Union: Volume 4, EU Guidelines to GMP for Human and Veterinary Medicinal Products, Annex 1, Manufacture of Sterile Medicinal Products; European Commission: 2008
Failure to properly adhere to the regulations may lead to observations. The FDA posts warning letters on its website (http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/). These letters can be reviewed for insight into the current regulatory expectations. This knowledge can then be utilized to prevent similar findings.
From reviewing the warning letters, it is evident there is a regulatory expectation to establish an adequate disinfectant and cleaning program which includes a sporicidal agent. For example, the following warning letter excerpts cite companies for this deficiency.
- Warning letter dated June 20, 2016: “In addition, your firm does not use sterile wipes, lint-free mop heads, or a sporicidal agent as part of the disinfection program to clean the ISO 5 hood and ISO 7 cleanroom where sterile drug products are prepared.”5
- Warning letter dated December 23, 2016: “Your cleaning and disinfection program lacked use of a sporicidal agent. ... A sound disinfectant program also includes a written schedule, sound methods, efficacy studies, and environmental data to support the ongoing effectiveness of the agents.”5
- Warning letter dated August 24, 2017: “Your firm failed to use disinfectant agents that are appropriate for use for cleaning the [redacted], the [redacted], and the [redacted] which are located inside the aseptic processing area. For example, you use non-sterile [redacted] and non-sterile wipes and do not use a sporicidal agent.” 5
- Many warning letters contain this statement: “Your firm failed to establish an adequate system for cleaning and disinfecting the room and equipment to produce aseptic conditions [21 CFR 211.42(c)(10)(v)].”5
Standard Industry Practice
Opinions have changed over the years, and rotating disinfectants has been debated again and again. Many industrial microbiologists believe the expectation to rotate disinfectants was originally extrapolated from the medical industry.6,3,7 It is widely known that doctors are worried about antibiotic resistance, and rightly so. However, this same phenomenon has not been observed in the field of cleaning and disinfection for the pharmaceutical industry.6,3,7 The extrapolation from the antibiotic resistance phenomenon to potential disinfectant resistance has triggered some companies to establish disinfectant rotation programs that are not scientifically sound.7 We need to identify the science behind the need for rotation.
Confusion has also grown from the various guidance and regulatory documents. For example:
- Annex 1 provides the following information: “The sanitation of clean areas is particularly important. They should be cleaned thoroughly in accordance with a written program. Where disinfectants are used, more than one type should be employed. Monitoring should be undertaken regularly in order to detect the development of resistant strains.”8
- USP <1072> states: “The development of microbial resistance to antibiotics is a well-described phenomenon. The development of microbial resistance to disinfectants is less likely to occur at significant levels, as disinfectants are more powerful biocidal agents than antibiotics. In addition, they are normally applied in high concentrations against low populations of microorganisms usually not growing actively, so the selective pressure for the development of resistance is less profound. However, the most frequently isolated microorganisms from an environmental monitoring program may be periodically subjected to use-dilution testing with the agents used in the disinfection program to confirm their susceptibility, as there are real differences among different species in resistance to the lethal effects of different sanitizers. … The rotation of an effective disinfectant with a sporicide is encouraged. It is prudent to augment the daily use of a bactericidal disinfectant with weekly (or monthly) use of a sporicidal agent. The daily application of sporicidal agents is not generally favored because of their tendency to corrode equipment and because of the potential safety issues with chronic operator exposure. Other disinfection rotation schemes may be supported on the basis of a review of the historical environmental monitoring data. Disinfectants applied on potential product contact surfaces are typically removed with 70% alcohol wipes. The removal of residual disinfectants should be monitored for effectiveness as a precaution against the possibility of product contamination.”2
- PDA TR 70 goes on to say: “Concerns for the possible resistance of organisms to these products are based on a theoretical relationship to resistance found with antibiotics. To date, there is no conclusive published test data proving such development of resistance by organisms to these agents. …”3
To gain a recent industry perspective, I polled 15 prestigious pharmaceutical microbiologists with a simple question: “Do you believe in disinfectant rotation?” I anticipated we would all agree. As a collective group, we have hundreds of years of experience in pharmaceutical microbiology. Table 1 summarizes the anonymous results of the polling.
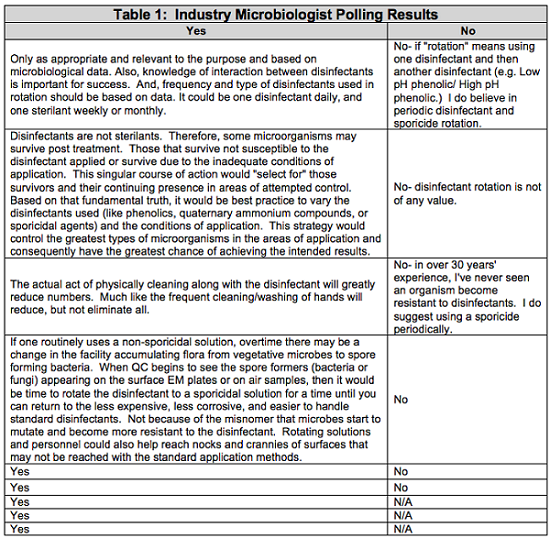
It was interesting that even the most prestigious industry microbiologists had somewhat varying opinions on disinfectant rotation. In fact, nine of the 15 microbiologists said they believe in the concept of disinfectant rotation.
One factor that may play a role in the varying opinions is the term “rotation” itself. Rotation is defined as a return or succession in a series.9 For example, a true rotation would be the use of Disinfectant A, then Disinfectant B, then Disinfectant A, then Disinfectant B, and so on.
Not all of the microbiologists who agree with disinfectant rotation believe the need for rotation is based on the theory of disinfectant resistance. Through discussions with industry microbiologists, another belief emerged. This theory is based on inherent resistance as opposed to acquired resistance. The acceptance requirements outlined in USP <51> were an example of this theory. Some disinfectants will not destroy every microorganism they encounter. Over time, the surviving microbial flora may be targeted based on their inherent resistance to these disinfectants, and not because they have mutated to acquire resistance. An example of inherent resistance was described in “Increasing Tolerance of Hospital Enterococcus faecium to Hand-wash Alcohols” by Pidot, S. et al. in May 2016.10
After compiling the results, I reflected on colleagues’ answers and explanations, my own opinion, the regulatory expectations and observations I researched, the industry guidance documents, and journal articles from industry experts. Literature research has demonstrated that true resistance or tolerance to common disinfectants has not been documented in the field to date. The common disinfectants attack microorganisms in various ways.6 The high levels of powerful, effective disinfectants versus the low level of microorganisms in a controlled environment provides swift microbial destruction.6 Thus, rotating between similar classes of disinfectants on the basis of preventing microbial resistance is not scientifically sound.7 A more effective practice includes the use of an effective disinfectant that is periodically switched out for a sporicidal agent. The residue from the chemical agents is subsequently removed with 70 percent isopropyl alcohol.11
Conclusion
In summary, I believe the current standard industry practice is to routinely use a qualified disinfectant, periodically supplement the disinfection process with a sporicidal agent, and regularly remove any residual residues with 70 percent isopropyl alcohol.2 This process is compliant, and many companies have proven it is effective in controlling microbial contamination.
Is this current industry practice considered a “rotation?” Some say, “yes,” others say, “no.”
While there is not a distinct answer on disinfectant rotation, the current industry practice is effective and widely accepted. Like many authors before me, I believe the confusion comes not only from the misinterpretation of the need to rotate disinfectants being based on the antibiotic microbial resistance phenomenon, but also from the term “rotation” itself.
Regardless of the terminology, there is a regulatory expectation to establish an adequate system for cleaning and disinfection in order to keep microbial contamination under control. The use of an effective disinfectant with a periodic shock to the environment with a sporicide is considered superior and is encouraged over the rotation of multiple disinfectants.3,11,2
Another consideration in establishing an adequate program includes the frequency between the switch of the routine disinfectant and the sporicidal agent. This frequency should be based on the results of microbial risk assessments, disinfectant efficacy results, EM trending results, and routine EM results.12
In my opinion, until the industry coins a better term than “rotation” for the current standard industry practice, the confusion over disinfectant rotation may continue. So, when regulators ask if you rotate your disinfectants, skip the “yes-or-no” debate. Clearly explain your cleaning and disinfection program, and then demonstrate through data how your program is effective in microbial contamination control.
Peer Review:
The author would like to thank the peer reviewers: Tony Cundell, Ph.D, William H. Miele, Ph.D., Mark Warner, M.S., and Julie Barlasov, MBA for reviewing this article and for their insightful comments and helpful suggestions.
References:
- PDA (2014). PDA Technical Report No. 13: Revised 2014: Fundamentals of an Environmental Monitoring Program. PDA, Bethesda, MD.
- USP <1072> Disinfectants and Antiseptics.
- PDA (2015). PDA Technical Report No. 70: Fundamentals of Cleaning and Disinfection Programs for Aseptic Manufacturing Facilities. PDA, Bethesda, MD.
- Sartain, E. (2005). The ABCs of Disinfectant Validation. March 2005. Accessed on August 31, 2018 at https://electroiq.com/2005/03/the-abcs-of-disinfectant-validation/.
- FDA Warning Letters. Accessed on August 31, 2018 at http://www.fda.gov/ICECI/EnforcementActions/WarningLetters/.
- Martinez, J. (2008). The Rotation of Disinfectants Principle: True of False. Pharmaceutical Technology, Vol. 33, Issue 2.
- Sutton, S. Ph.D. (2005). Disinfectant Rotation. A Microbiologist’s View. Controlled Environments, July 2005. Assessed on August 31, 2018 at http://www.microbiol.org/wp-content/uploads/2010/07/sutton.Controlled.Environ.2005.8.7.9.pdf.
- Annex 1 (2008). The Rules Governing Medicinal Products in the European Union — EU Guidelines to Good Manufacturing Practice, Medicinal Products for Human and Veterinary Use. Annex 1 — Manufacture of Sterile Medicinal Products. European Commission EudraLex, 4.
- Merriam-Webster Dictionary, rotation definition. Accessed on September 10, 2018 at https://www.merriam-webster.com/dictionary/rotation.
- Pidot, S. et al. (2016). Increasing Tolerance of Hospital Enterococcus faecium to Hand-wash Alcohols. Assessed on September 6, 2018 at https://www.biorxiv.org/content/early/2018/01/29/053728.
- Sartain, E. (2005). Disinfectant Rotation. R&D, March 2005. Assessed on August 31, 2018 at https://www.rdmag.com/article/2005/03/disinfectant-rotation.
- Azab, W. (2018). Lifecycle Approach to Cleaning and Disinfectant Rotation. Cleanroom Technology, March 2018. Accessed on August 31, 2018 at https://www.cleanroomtechnology.com/news/article_page/Lifecycle_approach_to_cleaning_and_disinfection_rotation/140448.
About the Author:
 Crystal M. Booth is a microbiology consultant. She has over 19 years of experience in pharmaceutical microbiology, working in quality assurance, CDMOs, R&D, and quality control laboratories, including startup companies. During her career, she has developed and validated methods for antibiotics, otic products, topical creams, topical ointments, oral solid dose products, oral liquid dose products, veterinary products, human parenterals, vaccines, biologics, aseptically filled products, and terminally sterilized products. Those methods include microbial limits testing, bacterial endotoxins testing, particulate testing, sterility testing, pharmaceutical water system validations, EM programs, surface recovery validations, disinfectant efficacy studies, minimum inhibitory concentration testing, antimicrobial effectiveness testing, hold time studies, and various equipment validations. Booth earned her bachelor’s degree in biology from Old Dominion University and her master’s in microbiology from North Carolina State University.
Crystal M. Booth is a microbiology consultant. She has over 19 years of experience in pharmaceutical microbiology, working in quality assurance, CDMOs, R&D, and quality control laboratories, including startup companies. During her career, she has developed and validated methods for antibiotics, otic products, topical creams, topical ointments, oral solid dose products, oral liquid dose products, veterinary products, human parenterals, vaccines, biologics, aseptically filled products, and terminally sterilized products. Those methods include microbial limits testing, bacterial endotoxins testing, particulate testing, sterility testing, pharmaceutical water system validations, EM programs, surface recovery validations, disinfectant efficacy studies, minimum inhibitory concentration testing, antimicrobial effectiveness testing, hold time studies, and various equipment validations. Booth earned her bachelor’s degree in biology from Old Dominion University and her master’s in microbiology from North Carolina State University.
