Planning & Executing Small-Scale Model Qualification For Upstream & Downstream Biopharma Processing
By Joschka Johannes Buyel (Bayer AG), Klaus Kaiser (Bayer AG), Kiran Andra (Alexion), Robert G. Luo (GlaxoSmithKline R&D), Alexis Henry (Biogen), Caroline Leveder (Sanofi R&D), Mark Richards (Eisai Inc.), Trish Connolly (BioPhorum Development Group)
This article is the second in a three-part series that summarizes the discussions and exchanges of a BioPhorum Development Group workstream on small-scale model justification. Their purpose is to provide reference documents for bioprocess professionals who will carry out small-scale model justification. In order to write the articles, surveys were conducted among BioPhorum workstream team members on the practices of conducting small-scale model justification. The results of the surveys are not presented in these articles but are available in an expanded BioPhorum white paper on the topic. Part 1 discussed the applications, including viral clearance, quality oversight, and design considerations, for justification of SSMs. This part covers the current opinion on how to execute small-scale model qualification for both upstream and downstream SSMs and analytical setup.
Selecting The Appropriate Small-Scale Model
When performing small-scale studies, an appropriate small-scale model that fits the model expectations has to be selected. For upstream unit operations, the benchtop-scale (1 L to 10 L) is currently the most prominent small-scale model used, but both micro-scale bioreactors (15 to 250 mL) and pilot-scale (50 to 200L) models are also applied.
Depending on the cell culture process, typically a scale-independent “scaling parameter” is selected in order to represent the manufacturing scale in the best possible way (Table 1). The current industry standard is to change scale-dependent model input parameters to best match large-scale outputs and to realize the intended scale-independent scaling parameter that is kept constant across scales (Table 1).
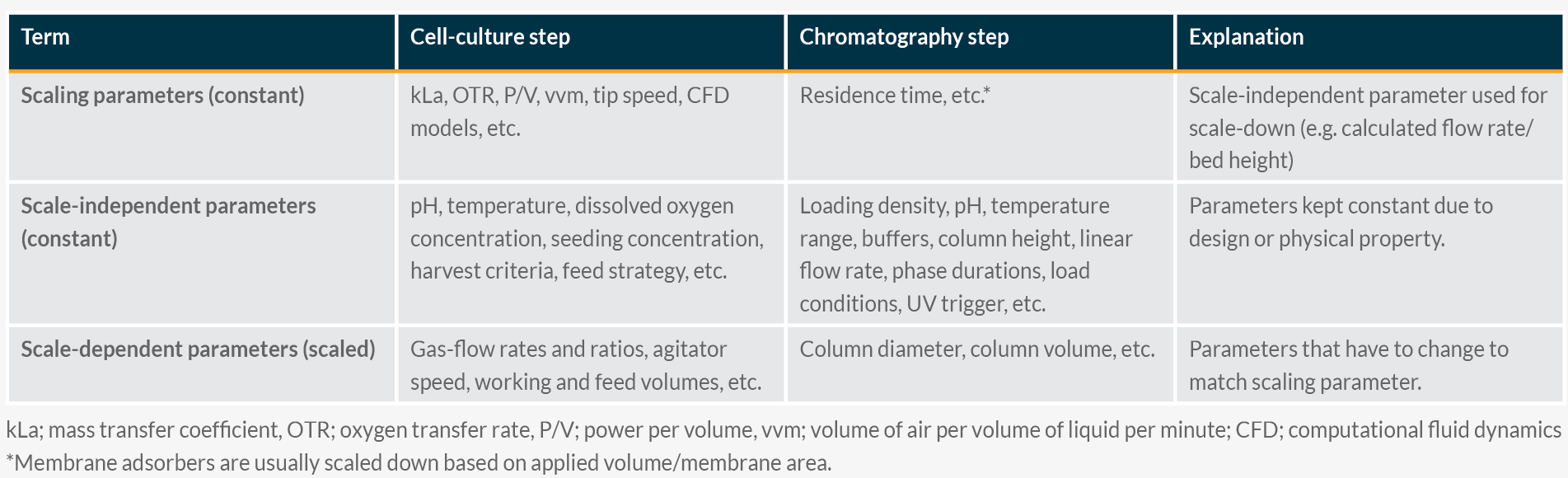
Table 1. Comparison of terms: Scaling Parameter, Scale-Independent Parameter, and Scale-Dependent Parameter. Click on image to enlarge.
For downstream, the benchtop scale of a chromatography column is typically operated by maintaining manufacturing-scale bed height and linear flow rate (cm/h) while performing a linear scale down. In cases where micro-scale (< manufacturing-scale column bed height) models are qualified, residence time is kept constant if linear velocity and bed height are demonstrated to not impact the outputs of the small-scale model (Table 1). Pilot scale can be used when appropriate.
Buffers and media can be prepared either at small scale or transferred from manufacturing scale. In some instances, the supply of buffers and media from manufacturing scale might be relevant for the representative behavior of unit operations.
For upstream unit operations, there may be some critical material attributes for which maintaining a consistent raw material lot across scales is recommended.
To supply downstream unit operations, buffers are usually prepared at the site performing the small-scale model qualification but can also be transferred from manufacturing scale. It may be useful to ensure use of the same or comparable procedures and raw materials for buffer preparation at small scale.
SSM Qualification Approach Upstream Processing
Most of the qualification efforts are focused on the production stage bioreactor as the most critical upstream unit operation in terms of impacting product quality and process complexity.
A formal qualification of the seed trains is usually not necessary because similar equipment and protocols are used between scales or the seed train is passively qualified by qualifying the performance qualification (PQ) of the production bioreactor.
For upstream unit operations, two typical SSMQ approaches are equally pursued:
In a satellite approach for each manufacturing-scale batch, at least one small-scale batch is inoculated from the same N-1 pre-culture material or transfer of an aliquot of material taken from the manufacturing-scale bioreactor shortly after inoculation (Figure 1). The feasibility of this approach depends on the close proximity of the small-scale and manufacturing-scale sites. It is generally accepted to use inoculum from single/few manufacturing runs to start multiple small-scale bioreactors.
The same batch/lot of media and solutions across scales shall be used. This paired satellite study design isolates the scale-dependent differences from random variability. Residual variability may be reduced and thus the capability to detect potential differences is enhanced.
A non-satellite approach where the seed train is performed at small scale may be preferred when inoculum transfer is not feasible or SSMQ work is uncoupled from large-scale runs. Both large scale and small scale define two independent data sets and do not require any transfers of inoculum, media, or solutions between the two scales (although this is not excluded).
The execution is less complex and does not require dedicated experiments. Data can be obtained from control runs generated during process characterization studies or DOE center points, assuming limited raw material lot-to-lot variability or same lots available at both scales. Other methods of comparison, such as multivariate analysis (MVA,) may also be used to qualify the model (see section 7.0 of the full paper).
Independent of how the SSM was qualified, it may be justifiable and beneficial to enable both non-satellite and satellite application of the same small-scale model for later use.
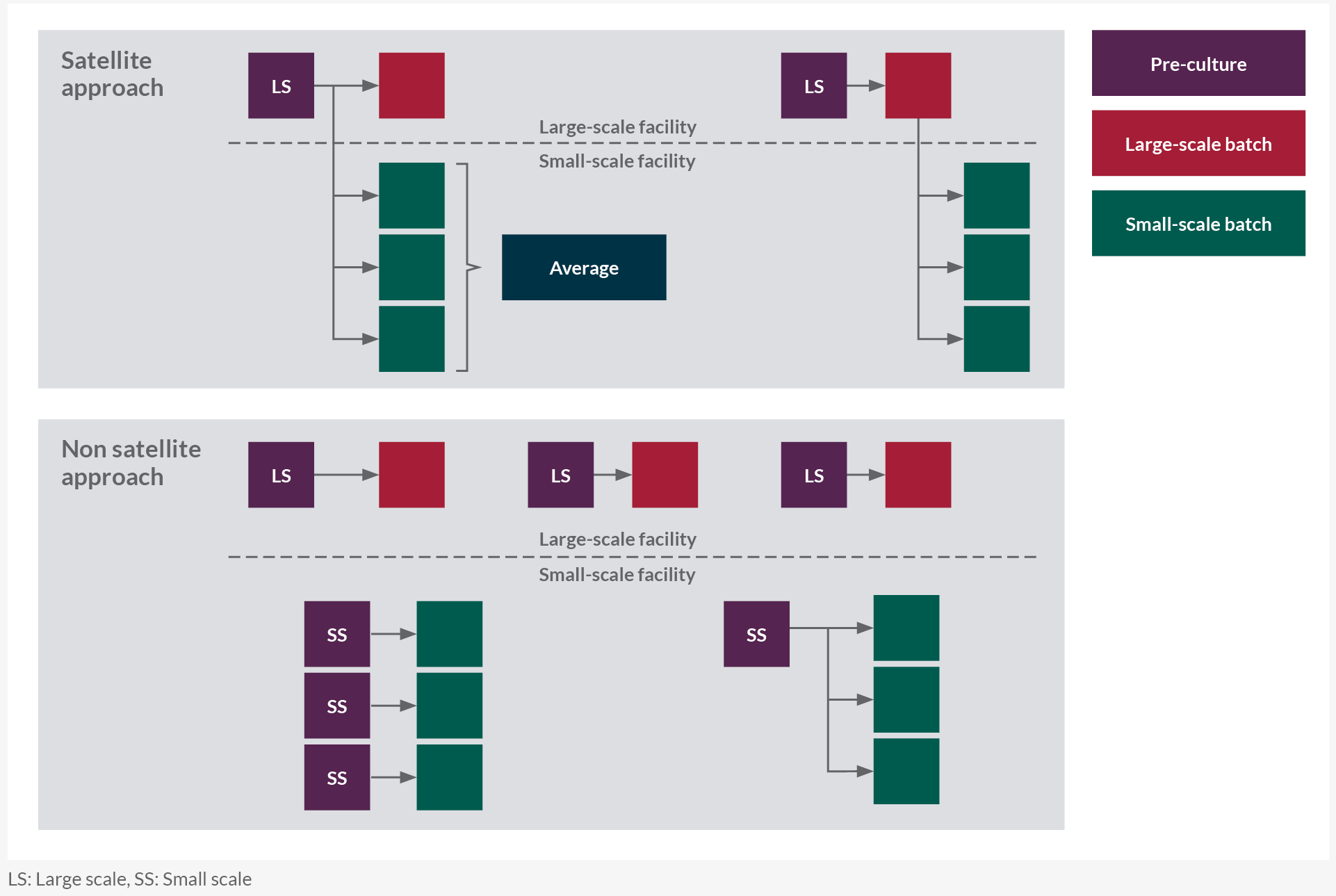
Figure 1: Satellite(A) versus non-satellite (B) approach.LS: Large scale, SS: Small scale. Click on image to enlarge.
During downstream SSM qualification, the small-scale runs are typically qualified against their respective large-scale run unit operations. Usually, load material produced in the previous step of the manufacturing process is utilized. Other load materials may also be used when the comparability of the load material to manufacturing-scale material was demonstrated.
Depending on the location of development laboratories and the commercial site, load material, intermediates, and samples may need to be stored or shipped. For most companies, shipping at ≤-65°C is the preferred condition but is highly dependent on the purity of the shipped intermediate as well as product-specific stability data available.
Small-scale model qualification is usually performed for all major unit operations where columns or membrane absorbers are used.
(Depth) filtration, centrifugation, or bulk fill steps are typically tested to be fit-for-purpose at smaller scale. All virus inactivation (low pH or solvent detergent) and virus filters cannot be qualified for their main purpose of viral clearance and are validated at small scale instead. These unit operations are only partially qualified for their impact on product quality and process performance based on a cause and effect investigation or sound scientific knowledge.
An ultrafiltration/diafiltration (UF/DF)step small-scale model can be limited in mimicking the manufacturing-scale physical conditions, yet representative parts of product quality and process performance can be assessed based on SME judgement.
Based on the transfer regime of load materials from manufacturing scale, a qualification approach is chosen (Figure 2). In a designed (paired) experiment, one or more small-scale runs are matched to each manufacturing-scale run. Most companies tend to use load material from one manufacturing batch for multiple (≥3) small-scale runs that are correlated and need to be averaged for qualification (Figure 2).
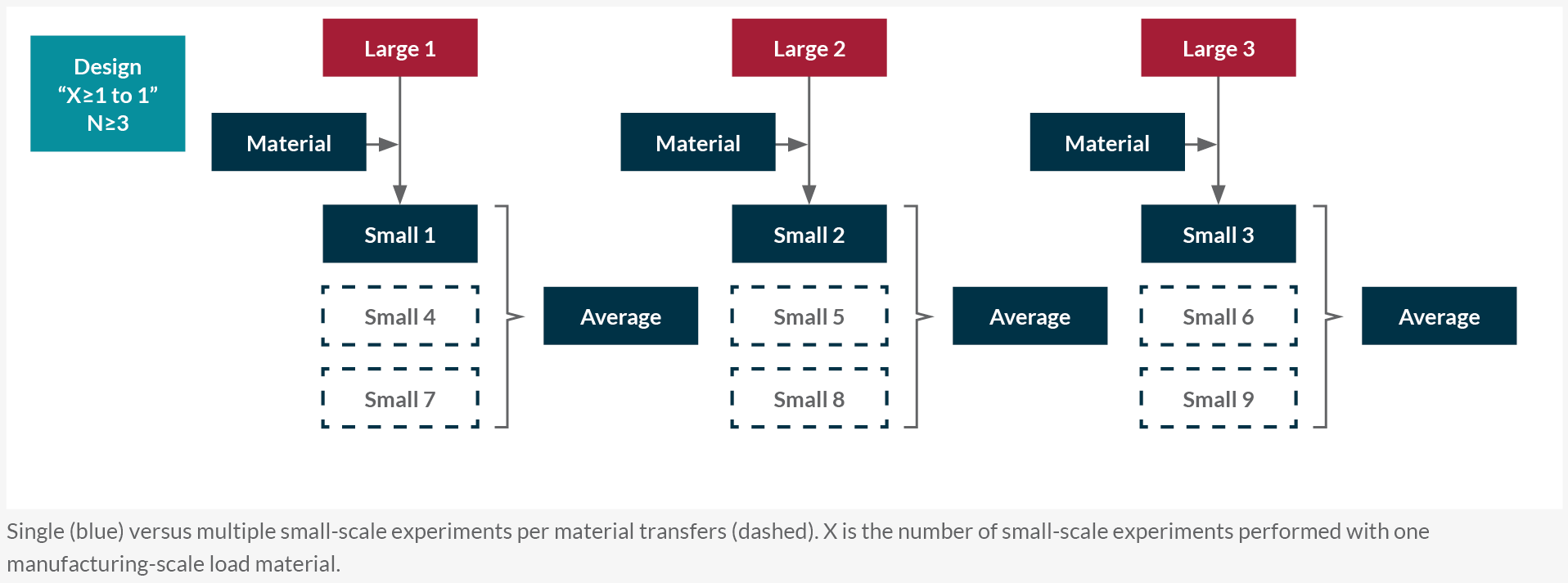
Figure 2: Typical downstream small-scale model qualification approaches. Single (blue) versus multiple small-scale experiments per material transfers (dashed). X is the number of small-scale experiments performed with one manufacturing scale load material. Click on image to enlarge.
It may be useful to assess the entire linked upstream and downstream process at small scale for final product quality. Some companies consider qualifying all small-scale model pools for the entire linked process against the corresponding manufacturing-scale pools. Other linked unit operations may be the N-1 and the production bioreactor, which are codependent, or virus inactivation at low pH that is performed with fresh capture eluate.
Analytical Setup
The analytical setup for small-scale experiments executed alongside the manufacturing scale should be stratified. The strategy relies on a risk-based exercise where critical quality and performance attributes are linked to particular unit operations.
Most manufacturing-scale samples are normally measured in QC GMP laboratories. Some small-scale experts also use QC GMP laboratory-generated data of SSM samples for small-scale model qualification. It may also be appropriate to perform non-GMP measurements of at-scale samples solely for the purpose of small-scale model qualification. Yet, the majority prefers an assay-dependent mixture of QC and non-GMP sample analysis for performing small-scale model qualification. When small- and manufacturing-scale samples are measured at different laboratories (including different sites and/or sample shipment), comparability between laboratories is usually demonstrated. When comparability is not confirmed, the minimum requirement is an assay-dependent fit-for-purpose exercise.
Batch testing artifacts could mask a true scale offset or introduce a false offset into a generated data set. Independent of the laboratory, batch testing artifacts (e.g., inter-assay sequence offsets mistaken for actual sample differences) should be avoided. A reference standard can be used to detect testing artifacts during routine batch measurements and control intermediate precision. Other measures, such as qualified assays and aligned sample preparation methods, shall be taken. It may also be of benefit to run small- and large-scale samples in one batch to better detect scale differences.
Operators
The operator training environment (e.g., trainings, gemba-walks, SOPs, best practices, protocols, automation, etc.) must ensure that small-scale unit operations can be performed independent of the operator. The majority of small-scale experiments are performed by development staff according to applicable guidelines.
Requalification Of The SSM
During the life cycle of a product, repetition of the qualification exercise may be required for various reasons (e.g., change in site, scale, or assay). Formal documentation should be considered in cases where comparability assessments and/or risk assessments are conducted to support the decision for not requalifying a model.
In Part 3, we will explore the statistical methods for comparing SSM outputs to at-scale outputs and cover descriptive statistical methods, inferential methods, difference tests, equivalence tests, quality range methods, and multivariate analysis.
Disclaimer: The approaches and methods presented in this paper resulted from surveys and discussions among team members from member companies. These approaches and methods do not in any way restrict member companies from using other approaches and methods they consider appropriate for their company’s studies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
