Experimental Methods For Microorganism Challenges On Online Water Bioburden Analyzers
By Cynthia Martindale (Applied Rapid Microbiology Specialists, Ltd.), Scott Hooper (Merck), Miriam Guest (AstraZeneca), Hans-Joachin Anders (Novartis), Ulrich Georg Zuber (Roche), and Mike Russ (Genetech)

Pharmaceutical and other water systems are required to produce water of a defined minimum microbiological quality. Classically, the ability of a system to meet the requirements has been demonstrated by growth-based enumeration of the microorganisms present in the water. Growth-based systems require time to observe growth, inherently resulting in a temporal delay between sampling and having a known result.
Online water bioburden analyzers (OWBAs) provide a faster (in fact, real-time) means of monitoring water system bioburden, and several commercial instruments are currently available for real-time rapid testing of microbial content in water systems. The continuous real-time monitoring offered by these instruments provides the user an opportunity to enhance the understanding of a water system in a way that cannot be achieved by traditional methods alone.1,2,3
Implementing OWBA technology often includes laboratory microorganism challenge testing to verify that the instrument is suitable for its intended use.4,5 Executing microbial challenge studies require the end user to select microorganisms for testing and successfully create quantifiable suspensions of these microorganisms for OWBA analysis. While this would seem to be a straightforward task, several aspects of preparing microbial challenges can easily derail the best intended experimental design.
This two-part article series presents information on executing microorganism challenge studies in a laboratory setting. This first article discusses two different experimental approaches that have been successfully used for microorganism challenges. The second article will discuss best practices to adhere to and pitfalls to avoid during execution of microorganism challenge tests.
Two Experimental Approaches for OWBA Microorganism Challenges
OWBAs are generally envisioned and designed as instruments where water from a source flows continuously through the instrument (termed online measurement) and is directly analyzed by the OWBA. Additionally, OWBAs can be used to analyze discrete water samples from containers (discrete sample testing).
Microorganism challenges conducted in a laboratory can use either analysis mode on an OWBA, as both modes allow the user to make controlled sample introductions into an OWBA for the purpose of enumerating defined microbial challenge samples. In discrete sample testing, containers are inoculated with a known concentration of microorganisms and introduced to the OWBA using the instrument pump. In online testing, a known concentration of microorganisms is injected into the water stream feeding into the OWBA.
Of the two microbial challenge approaches, discrete sample testing is the easiest to set up and does not require the use of specialized equipment for execution; however, material preparation is longer, as many sample containers must be individually prepared before use.
Conversely, online testing is more complicated with respect to initial set up and requires the use of some specialized equipment (e.g., syringe pumps); however, there are a minimal number of sample containers to be prepared/rinsed before use, and, therefore, it is less labor intensive to execute.
Despite having a more complicated up-front setup, online testing is more representative of the proposed routine application of this technology, and it provides the most reliable and consistent results. This is because the online test design utilizes a single sample preparation activity, whereas the discrete sample test design requires multiple sample preparations, resulting in experimental variability.
|
Advantages |
Discrete Sample Testing |
On-line Testing |
|
Easiest material preparation (lowest labor) |
|
x |
|
No up-front test system setup |
x |
|
|
Limited additional equipment needed |
x |
|
|
Reliable and consistent results |
|
x |
1. Microorganism Challenge Testing Using Discrete Samples
The OWBA is set to analyze individual containers with microorganisms suspended in water. The preparation of a discrete test sample for analysis (shown in Figure 1) starts with filling a container with water (1). This “water only” sample is tested by the OWBA (2).
One of the complicating factors in any OWBA measurement is ensuring that extraneous particulates do not masquerade as microbial counts; therefore, counts from this “water only” sample should be much lower than the theoretical colony forming unit (CFU) of the microorganism inoculum that is being prepared the OWBA counts during testing are related to the microorganism rather than the materials (e.g., water or sample container). Where OWBA counts are too close in number to the concentration of the test microorganism, the container should be rinsed and refilled, and new OWBA counts should be obtained.
Once the “water only” count(s) are satisfactory, the container is inoculated with the prepared challenge microorganism inoculum at a volume necessary to achieve the desired test concentration (3). The inoculated test container is mixed gently for homogeneity and tested using the OWBA (4). Once the OWBA testing had been completed, the remaining sample volume is used to complete conventional plate count testing (5).
A unique sample is prepared for each microorganism concentration. For each concentration, the microorganism inoculum preparation is used to inoculate a specified amount of water to achieve the desired theoretical CFU within each test container. Each of the prepared samples is tested as described above. Microorganism inoculum concentrations may be determined from the inoculum preparation each time a new test sample is prepared or at the end of testing.
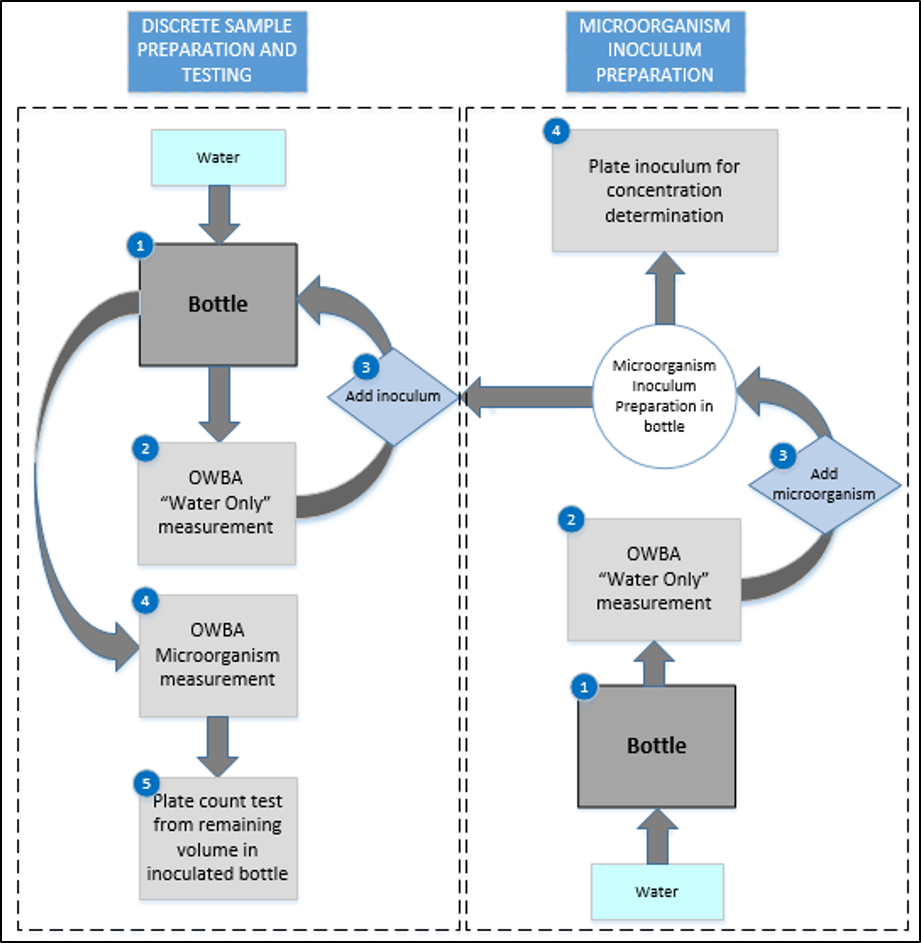
Figure 1: Experimental design for discrete sample microbial challenge testing
2. Microorganism Challenge Testing Using Online Testing
Online testing requires that a water source continuously feeds the OWBA throughout the experiment. Refer to Figure 2 for a diagram where laboratory equipment is set up to achieve online injections of a microorganism. Although not depicted in Figure 2, it should be noted that the injection design may require the use of check valves or pressure regulation to ensure the flows of water and microorganism are moving towards the OWBA and not backward into the water source or the syringe.
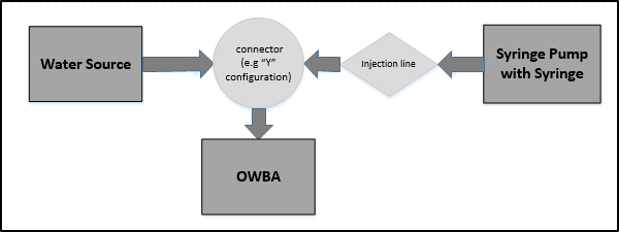
Figure 2: Setup of equipment for online microbial challenge testing
The water line merges with a line for the microorganism injection. A syringe filled with the microorganism preparation and is then connected to a syringe pump, which is used to control the rate of the injection. The number of microorganisms delivered to the OWBA is changed by increasing or decreasing the rate of injection on the syringe pump.
Prior to executing the online testing, an appropriate testing scheme should be developed so that for each microorganism concentration desired, the necessary syringe injection rates (volume per minute) can be calculated. The injection rate of the syringe can be calculated using the following equation:

The execution of an online microorganism challenge is shown in Figure 3.
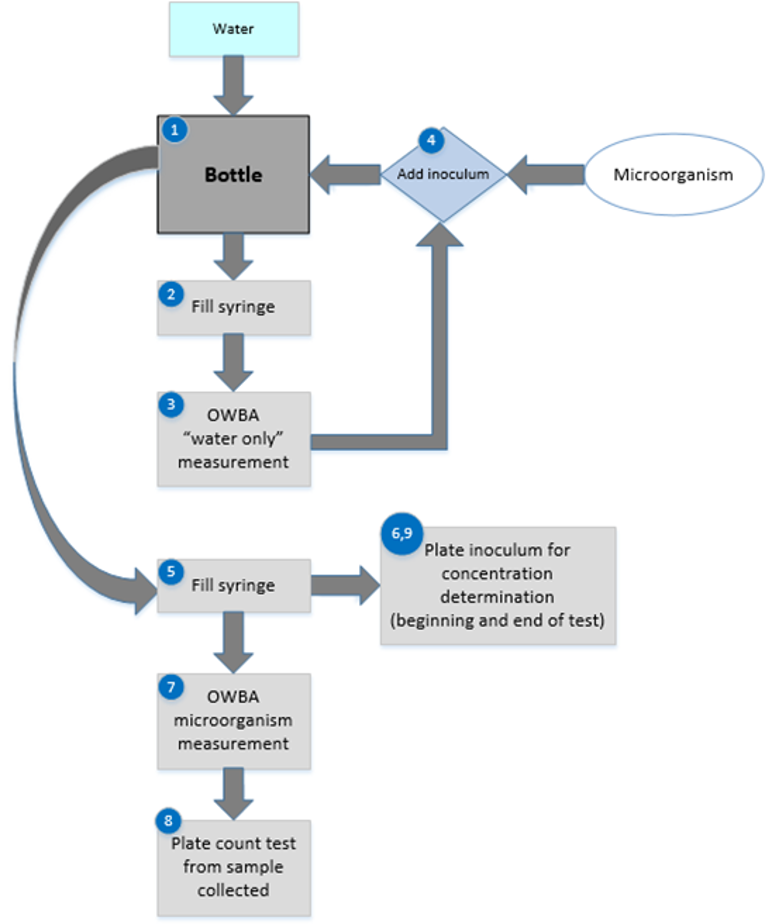
Figure 3: Experimental design for online microbial challenge testing
On the day of the test, a container is filled with water (1) and then used to subsequently fill a syringe (2). The syringe is secured to a syringe pump and the injection line is secured (refer to Figure 3). “Water only” counts by the OWBA are taken at each injection rate to be used in the testing scheme (3). For each injection rate, if the measured count(s) is too close in number to the concentration of the test microorganism, the sample container and syringe should be rinsed and refilled, and new OWBA counts should be obtained.
Once all the measurements at all the injection rates of the “water only” have been satisfactorily completed, the water remaining in the container is inoculated with the prepared challenge microorganism inoculum (4). The inoculated container is mixed gently for homogeneity and used to fill the syringe (5). A plate count to determine the inoculum concentration at the beginning of the test may be conducted by expelling a portion of the filled syringe into a sterile container (6).
The syringe containing the microorganism inoculum preparation is secured to a syringe pump and the injection line is secured. The syringe pump is set to the first injection rate and started. Counts of the microorganism are taken from the OWBA (7). A sample of the OWBA test material is also taken, from which plate count testing is conducted. In some cases, this sample may be drawn from a sanitized OWBA waste line.
For each injection rate to be tested, the injection rate is started, OWBA counts are taken, and a sample is collected for plate count testing. At the completion of the test, a plate count to determine the inoculum concentration may be conducted by expelling a portion of inoculum remaining in the syringe into a sterile container (6).
Conclusion
OWBAs can readily enumerate microorganisms in water with essentially real-time detection. Their increased sensitivity and differing method of detection of microbes as compared to classical growth-based methods means that OWBA studies require additional care to avoid introducing artifacts into the tests. The online and discrete sample testing approaches provide practical guidance for OWBA users to create and conduct their own controlled studies of water using online bioburden analyzers. In the next article of this two-part series, we will explore best practices and pitfalls when conducting microorganism challenges.
References:
- Cundell, A., O. Gordon, N. Haycocks, J. Johnston, M. Luebke, N. Lewis, J. Mateffy, and J. W. Weber. 2013. Novel Concept for Online Water Bioburden Analysis: Key Considerations, Applications, and Business Benefits for Microbiological Risk Reduction Amer. Pharm. Rev. https://www.americanpharmaceuticalreview.com/Featured-Articles/140513-Novel-Concept-for-Online-Water-Bioburden-Analysis-Key-Considerations-Applications-and-Business-Benefits-for-Microbiological-Risk-Reduction/
- Anders, H.-J., F. Ayers, B. Fitch, R.-Y. Forng, S. Hooper, M. Luebke, J. Mateffy, P. Noverini, B. Termine, L. Yan, and J. Weber. 2017. Practical Application of Online Water Bioburden Analyzers in Pharmaceutical Manufacturing. Pharmaceutical On-Line [24-Jul-2017] http://www.pharmaceuticalonline.com/doc/Practical-application-of-online-water-bioburden-analyzers-in-pharmaceutical-manufacturing-0001
- Ayres, F., J.-P. Chen, M. Dingle, S. Hooper, L. Lawson, D. Manzer, P. Noverini, A. Prasad, A. Scott, P. Villari and J. Weber. 2019. Biofluorescent Particle Counter-Based Real-Time Feedback and Control of Processing Conditions. Eur. Pharm. Review In-Depth Focus: QA/QC & Analytical Techniques: Environmental Monitoring. 24:2-5.
- General Chapter <1223> Validation of Alternative Microbiological Methods, USP41/NF36, U.S. Pharmacopeia 2018.
- General Chapter 5.1.6 Alternative Methods for control of Microbiological Quality. European Pharmacopoeia 9.4, European Directorate for the Quality of Medicines and Healthcare (EDQM), Strasbourg, Cedex, France: 2017.
