Cleaning Process Development: Selection Of Cleaning Agents For Pharmaceutical Products
By Ruijin Song and Andrew Walsh, Center for Pharmaceutical Cleaning Innovation (CPCI)
Part of the Cleaning Validation for the 21st Century series
Part 1 of this series on cleaning process development discussed how quick and economical laboratory-scale studies can be used to determine the "hardest-to-clean" products or compounds.1 In that article we also proposed that laboratory-scale studies can provide answers to typical cleaning validation questions such as:
- Which product is the hardest-to-clean?
- Which cleaning agent provides the best cleaning?
- Can we demonstrate that two cleaning agents are equivalent?
- How long does it take to clean the equipment?
- Which cleaning input parameters are critical?
- What are the optimal cleaning parameter settings?
- Are dirty hold time studies necessary at commercial scale?
This article will discuss how bench-scale studies can be used for selecting the best cleaning agent for certain products and provide answers to Questions 2 and 3.
Question 2: Which Cleaning Agent Provides The Best Cleaning?
The rationale for selecting a cleaning agent is of great importance in cleaning validation. Cleaning agents cannot be randomly chosen and must be shown to be appropriately chosen and effective.
From a patient perspective, cleaning agents that are used in a cleaning process should be innocuous and as nontoxic as possible so any residues the patient may be exposed to will cause no harm. From a company perspective, cleaning agents must be very effective in reducing residues to acceptable levels as cleaning times can be very long impacting operational efficiencies and the cleaning agents themselves are quite expensive and can incur unnecessary costs.
The composition of a cleaning agent should be known, in order to evaluate its hazard level, which may require making formal inquiries to the manufacturer. Where health-based exposure limits (HBELs) are significantly high and the risk to patient safety and product quality is significantly low, testing for cleaning agent residues may not even be necessary. Where HBELs are significantly low and the risk to patient safety and product quality is significantly high, testing for cleaning agent residues is necessary and the detectability of cleaning agent residues must be considered.
Cleaning agent selection should also be based on their suitability for the product they are intended to clean.
There are many different types of products that leave different residues that require different cleaning processes, such as:
- Simple (aqueous) solutions
- Gels/creams/ointments
- Powders/granulations/ tablets/capsules
- Protein/peptide/nucleic acid preparations
The cleaning agent selected should be appropriate for the residue being cleaned. There are many types of cleaning agents being promoted to the pharmaceutical industry, including alkali-based, acid-based, detergent-based, etc. How do you determine which cleaning agent is the most appropriate? There are existing ASTM (American Society for Testing and Materials) standards that can specifically be used for this purpose.
As discussed in a previous article,2 two newly updated ASTM standards for the pharmaceutical and medical device industries can be used for determining a cleaning effectiveness factor (CEF) of compounds or products. This article showed how compounds or products can be prepared for testing in the laboratory by spiking the compounds or products onto test "coupons" following the ASTM G121 Standard Practice3 and how they can be tested using standard gravimetric analysis following ASTM G122 Standard Method for evaluating cleaning agents and processes4. This CEF is a measure of the cleanability of compounds or products from pharmaceutical manufacturing equipment surfaces or medical device surfaces.
Selection Of Cleaning Agents Using Cleanability Testing
Bench-scale cleanability studies as described in Part 1 of this series on Cleaning Process Development can also be used for selecting the best cleaning agent for a product or a group of products. Figure 1 shows the comparison of four hypothetical cleaning agents for reducing residues of hypothetical product A. Agents 1 and 2, which have the highest CEF levels (meaning more residues remain) are the least effective cleaning agents for this product. The CEF level for Agent 3 is much lower (meaning less residue remaining) than for 1 and 2, so this is a more effective cleaning agent. However, Agent 4 appears to have the lowest CEF and may be the most effective cleaning agent for product A.
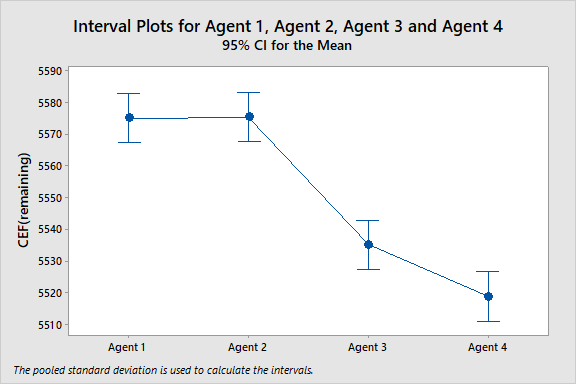
Figure 1: Interval plots for four hypothetical cleaning agents
Since there are several sets of data, an analysis of variance (ANOVA) was also performed for these cleaning agents. The ANOVA had a P-Value = 0.000, meaning that at least one mean is different from the others, and these cleaning agents should not be considered equivalent. Unfortunately, ANOVA on its own is not very helpful in making decisions about which cleaning agent may be the best. But there are several additional statistical tests (Dunnett’s and Hsu's) that can be performed on top of the ANOVA analysis that can provide better insight into the evaluation.
Dunnett's method is used in ANOVA to create confidence intervals for the differences between the mean of each factor level and the mean of a control group. If an interval contains zero, then there is no significant difference between the two means under comparison. You specify a family error rate for all comparisons, and Dunnett's method determines the confidence levels for each individual comparison accordingly. We can see in the analysis in Figure 2 that there is no difference between Agent 1 and Agent 2, but there is a large difference between Agent 1 and Agent 3 and an even larger difference between Agent 1 and Agent 4. This could be visually inferred from the interval plots of the ANOVA in Figure 1, but Dunnett's test provides a more objective confirmation.

Figure 2: Dunnett's method
Hsu's method allows you to pick either the highest or lowest mean as the "best" mean and compare it to the others to determine which are significantly, or insignificantly, different from the "best." Hsu's method can be used after an ANOVA to more precisely analyze differences between the means. It is usually impossible to find one cleaning agent that is suitable for all products. This test method can provide an efficient way to help us choose the best cleaning agent for different products and thus improve the efficiency and quality of cleaning processes. In the analysis in Figure 3, the cleaning agent with the lowest remaining residue ("best") is Agent 4 and we can see that the means for Agents 1, 2, and 3 are all significantly higher (worse) than the mean for Agent 4. This comparison also shows that Agent 4 is significantly lower than Agent 3. So, Agent 4 is the best cleaning agent for the product being tested.
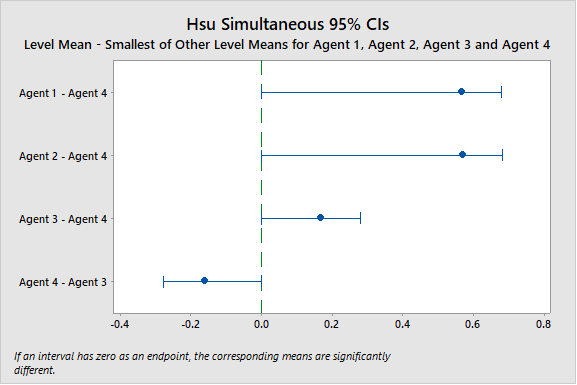
Figure 3: Hsu's multiple comparison method
By using statistical tools such as Minitab or others, we are able to look more deeply into the nature of the data and their relationships so that we can may draw more scientific conclusions and make the right decisions.
Question 3: Can We Demonstrate That Cleaning Agents Are Equivalent?
To determine the “hardest-to-clean” product, we used the two-sample t-test or ANOVA. These statistical methods are used to show that there are differences between the means of data but cannot be used to demonstrate equivalency. These tests are based on the null hypothesis, where, if we cannot show that there is a difference, we "accept" that the null hypothesis is true and that there is no difference. Note, however, that these tests cannot demonstrate that the null hypothesis is true and that there truly is no difference.
One of the operational issues that has arisen out of traditional cleaning validation studies is that after the validation is completed, the conditions of the validation become "locked." That is, they cannot be changed. This has included the cleaning agent. Once a cleaning agent has been selected and the cleaning process is validated, the choice of cleaning agent is considered fixed and cannot be changed easily. Otherwise, another full cleaning validation must be performed, and this is quickly rejected, as the amount of work is too costly and, more importantly, the fear of a revalidation failure is strong. The use of the two one-sided t-test (TOST) with cleanability test data can provide a high degree of assurance that two different cleaning agents are equivalent and that one cleaning agent could be substituted for the other cleaning agent.
To demonstrate that the means of two cleanability tests for two cleaning agents are equivalent, we need to use an acceptable two-sample equivalence test, which evaluates whether the mean of a "test population" is equivalent to the mean of a "reference population." For this test, an equivalence interval, or zone of equivalence, is chosen as a boundary for any differences between the data sets. This equivalence interval, which is chosen on your understanding of the products, or test procedure, must be chosen before you can run the test. The two-sample equivalence test then determines whether any difference between the two means is within the equivalence interval you have chosen. This is also known as a TOST and has already been used to show equivalency of cleanability data.5
Figure 4 shows the results of an equivalence test for Cleaning Agent 1 and Cleaning Agent 2 from Figure 1. In Figure 1, the 95% confidence intervals for these two cleaning agents looked almost identical. Since the range of the sample data for Cleaning Agent 1 was 10, this value was chosen for the equivalence interval, which is quite tight.

Figure 4: Two-sample equivalence test of Cleaning Agent 2 and Cleaning Agent 1
Figure 5 shows the results of an equivalence test for Cleaning Agent 3 and Cleaning Agent 4 from Figure 1. In Figure 1 the 95% confidence intervals for these two cleaning agents looked different but might overlap, indicating they might possibly be equivalent. The equivalence test evaluates whether the mean of a "test population" (Agent 2) is equivalent to the mean of a "reference population" (Agent 1). The range of the sample data for Cleaning Agent 1 was 10, so this test used a very tight criteria of ± 10 for the equivalence interval to show equivalence.

Figure 5: Two-sample equivalence test of Cleaning Agent 4 and Cleaning Agent 3
In this case, the mean of Cleaning Agent 3 is outside the equivalence interval for Cleaning Agent 4 and is not equivalent to Cleaning Agent 4. These two cleaning agents should not be considered equivalent.
Summary
It is becoming important that a substantial amount of bench-scale testing and analysis should be done early in cleaning process development, as described in ASTM E3106 Standard Guide for Science-Based and Risk-Based Cleaning Process Development and Validation,6 to provide data on cleanability testing to:
- Determine the “hardest-to-clean” products
- Evaluate and select cleaning agents
- Determine critical cleaning process parameters (design of experiments)
- Determine “time to clean” and the "cleaning assurance level"
- Evaluate the need for dirty hold time studies
- Evaluate new products against current cleaning agents/cleaning procedures and predict cleaning performance
The "cleanability" of pharmaceutical products has been defined as "the relative ease or difficulty for cleaning a product"3 and is a major factor in the performance of pharmaceutical cleaning processes. Cleanability is also a key parameter for consideration in design of experiments and building "cleaning design space" and plays a central role in quality by design for cleaning processes. The CEF, which indicates the fractional contaminant that remained or was removed during cleaning, is calculated from actual test data and can be used as a measurement of cleanability.
Cleanability testing combined with statistical equivalence tests can be used to select the most effective cleaning agents or demonstrate that two cleaning agents are equivalent. This process knowledge, along with perhaps a single qualification or verification run supported by ongoing verification (TOC or even just visual inspection), can simplify the exchange of one cleaning agent for another or justify the use of more than one cleaning agent, providing savings in time, money, energy, and resources.
Another important consideration for the selection of cleaning agents is the level of risk that any residues of their components present to patients. Any cleaning agents used for pharmaceutical manufacturing equipment or medical devices should be safe to use. A subsequent article will discuss a procedure for evaluating the safety of cleaning agents and whether they can be safely used.
Peer Review:
The authors wish to thank Sarra Boujelben, Gabriela Cruz, Ph.D., Mallory DeGennaro, Parth Desai, Ioana Gheorghiev, M.D., Miquel Romero Obon, Prakash Patel, Siegfried Schmitt Ph.D., and Joel Young for providing many insightful comments and helpful suggestions.
References:
- Song, Ruijin, Alfredo Canhoto, and Andrew Walsh "Cleaning Process Development: Cleanability Testing And "Hardest-To-Clean" Pharmaceutical Products" Pharmaceutical Online , January 2019
- Song, Ruijin and Andrew Walsh "ASTM Standards for Cleanability Testing of Pharmaceuticals and Medical Devices" Pharmaceutical Online, September 2020
- ASTM G121 “Standard Practice for Preparation of Contaminated Test Coupons for the Evaluation of Cleaning Agents” https://www.astm.org/Standards/G121.htm
- ASTM G122 "Standard Test Method for Evaluating the Effectiveness of Cleaning Agents and Processes” https://www.astm.org/Standards/G122.htm
- Chen, C., et al., “Statistical Equivalence Testing for Assessing Bench-Scale Cleanability,” BioPharm International, February 2010, Vol. 23, Issue 2, www.biopharminternational.com
- ASTM E3106-18 “Standard Guide for Science-Based and Risk-Based Cleaning Process Development and Validation” https://www.astm.org/Standards/E3106.htm
About The Authors:
 Ruijin Song is a senior validation specialist at the Center for Pharmaceutical Cleaning Innovation (CPCI), where she has been engaged in research on cleanability studies, including determination of hardest-to-clean products, cleaning agent selection studies, time-to-clean studies, and the design of experiments to optimize cleaning processes for internal research and CPCI clients. She also has extensive experience in TOC and UV method development and validation, swab recovery studies, and the qualification of visual inspection. Song has given two presentations at industry conferences and one publication on cleaning process development. She has an MS in chemical engineering from Columbia University and an MS in material science from Tongji University. Song can be contacted at ruijin.song@clean6sigma.com.
Ruijin Song is a senior validation specialist at the Center for Pharmaceutical Cleaning Innovation (CPCI), where she has been engaged in research on cleanability studies, including determination of hardest-to-clean products, cleaning agent selection studies, time-to-clean studies, and the design of experiments to optimize cleaning processes for internal research and CPCI clients. She also has extensive experience in TOC and UV method development and validation, swab recovery studies, and the qualification of visual inspection. Song has given two presentations at industry conferences and one publication on cleaning process development. She has an MS in chemical engineering from Columbia University and an MS in material science from Tongji University. Song can be contacted at ruijin.song@clean6sigma.com.
 Andrew Walsh is president of CPCI, a not-for-profit research and educational organization focused on cleaning validation. He is very active in developing industry consensus standards with ASTM and has led the teams writing the E3106 Standard Guide for Cleaning, the E3219 Standard Guide for HBELs, the G121 Standard Practice for Preparation of Coupons, the G122 Standard Method for Evaluating Cleaning Agents and Processes, and the E3263 Standard Practice for Qualification of Visual Inspection. Walsh is also involved with updating the PDA Technical Report #29 on cleaning validation. He is also writing a textbook on science, risk, and statistics-based cleaning process development and validation. He teaches a graduate-level course in cleaning validation in the Temple University School of Pharmacy Regulatory Affairs and Quality Assurance Program. Walsh has an MS in biology (microbiology) and is a certified Lean Six Sigma Black Belt and an Accredited Trainer. He can be contacted at andywalsh@clean6sigma.com.
Andrew Walsh is president of CPCI, a not-for-profit research and educational organization focused on cleaning validation. He is very active in developing industry consensus standards with ASTM and has led the teams writing the E3106 Standard Guide for Cleaning, the E3219 Standard Guide for HBELs, the G121 Standard Practice for Preparation of Coupons, the G122 Standard Method for Evaluating Cleaning Agents and Processes, and the E3263 Standard Practice for Qualification of Visual Inspection. Walsh is also involved with updating the PDA Technical Report #29 on cleaning validation. He is also writing a textbook on science, risk, and statistics-based cleaning process development and validation. He teaches a graduate-level course in cleaning validation in the Temple University School of Pharmacy Regulatory Affairs and Quality Assurance Program. Walsh has an MS in biology (microbiology) and is a certified Lean Six Sigma Black Belt and an Accredited Trainer. He can be contacted at andywalsh@clean6sigma.com.
