Best Practices For Cell Culture Media Fingerprinting
By BioPhorum
Cell culture media range from simple components to complex chemically defined mixtures. They may contain many chemical components, each with individual properties, where any variation may impact the cell culture process and the product being manufactured. While simple substances can easily be identified using well-known classical methods (such as Fourier transform infrared spectroscopy), this becomes more challenging when dealing with complex mixtures.
Industry processes have been improved to ensure good manufacturing practices and controls are in place. One way this is achieved is by moving away from using non-chemically defined raw materials. A media user cannot quantify every media component and must find an appropriate technique for the intended use and accept a controlled risk of some product variation. If the critical components of a medium are unknown, a suboptimal source or raw material could be used and may result in the loss of batches. This could be expensive and significantly impact schedule and market supply.
The International Council for Harmonisation’s Q7A Good Manufacturing Practice Guidance for Active Pharmaceutical Ingredients Guidance for Industry (Section VII-3-C) requires biopharma end users to perform at least one test to verify the identity of each batch of material in addition to using a supplier’s certificate of analysis. Therefore, many end users duplicate the basic ID fingerprinting test performed by media manufacturers as part of their internal quality control processes. This duplication does not add any value to the fingerprint of the medium.
Many of us in industry recognize that the key challenges include:
- Lack of standard guidance for fingerprinting cell culture media and associated raw materials.
- Current testing may indicate formulation or quality attributes but not process impact/fit for purpose.
- Current ID methods for fingerprinting media formulations are cumbersome and often require different analytical techniques, e.g., high-performance liquid chromatography.
Our three-tier approach to media fingerprinting that we describe in this article helps you to define an alternative method that will allow verification of the quality of the medium as well as fingerprinting. It helps you to choose suitable methods for incoming ID testing based on raw material properties, goals of media characterization, and the resources available at the site of the media manufacturer or user. It is based on the basic principle of avoiding duplicate testing so that media users can trust the data provided by media manufacturers.
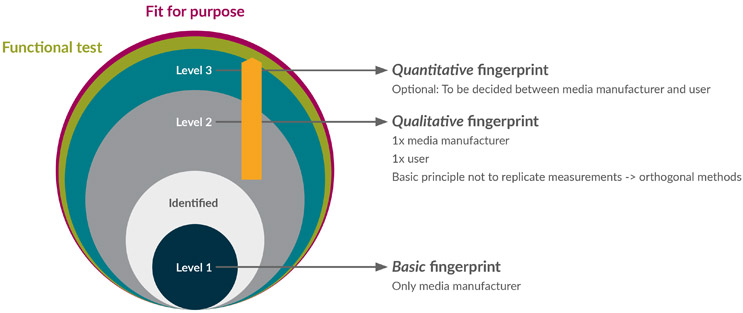
Figure 1: Three-tier testing approach to media fingerprinting.
In this context:
- It is the responsibility of the media supplier to perform Level 1 Basic (ID) testing and one type of Level 2 Qualitative fingerprinting to confirm there is no discrepancy in the identity or quality of the media provided.
- The end user is advised to perform Level 2 Qualitative fingerprinting to add dimensionality to the characterization of the material. This test should not be the same as the Level 2 Qualitative fingerprinting completed by the media supplier.
- Based on process knowledge and risk assessment of the material, Level 3 Quantitative fingerprinting for specific compounds might be warranted. This can be performed by the supply partner as an additional service or directly by the end user, depending on resources and the relationship between them.
Below, we describe the expectations of each level in the testing approach. This aim is to confirm that the cell culture media is fit for purpose and that the level of fingerprinting applied results from process knowledge and requirements.
Level 1: Basic (ID) Fingerprinting
This is defined as being able to distinguish one media from another at a gross level. What is being described will not uniquely identify each chemically defined media; rather, it can allow differentiation of media A from media B.
Physicochemical test methods are commonly used for Basic (ID) fingerprinting. These are simple techniques that indicate that the media has been accurately formulated and there are no gross errors in the overall composition.
Level 2: Qualitative Fingerprinting
Fingerprinting includes various methods that are fast and generally qualitative.
Spectroscopy, including infrared (IR), Raman, 2D-fluorescence, and total reflection X-ray fluorescence combined with multivariate data analysis, are often considered. Due to their higher cost, complexity, and environmental impact, chromatographic techniques are currently not often used for fingerprinting. However, there are examples of using liquid chromatography-mass spectrometry (LC-MS)/mass spectrometry (MS) for cell culture media fingerprinting. As technology evolves, the wider use of chromatography methods for Level 2 Qualitative fingerprinting can be considered.
Spectroscopic methods give information about the way that matter and individual analytes interact with electromagnetic radiation. This allows for collecting specific fingerprints of chemical compounds or mixtures of these. IR is the most established method followed by Raman, and both have shown they are suited for identifying simple compounds.
Two-dimensional fluorescence has been developed and successfully applied to fingerprinting cell culture media. Its potential for the fingerprinting of complex cell culture media raw material has been shown in research, and it has been applied to cell culture media characterization.
None of these methods can detect all compounds in a complex cell culture media sample, but they can provide enough information about the compounds to support a robust characterization or ID approach. Unfortunately, there is no clear library of references providing the specific extent of analytes that can be covered by each method. The guidance provided here supports identifying the method most appropriate depending on characterization goals, property of materials sample, or expertise available.
In theory, Raman and IR are sensitive to most chemicals; however, these methods will only be sensitive enough to detect the compounds present in higher concentrations in the media that, in most cases, will be predominantly buffer and amino acids. We do not expect that low-level vitamins or trace elements can be detected by these methods.
See Figure 2 for a decision-making chart for selecting a spectroscopic technique.
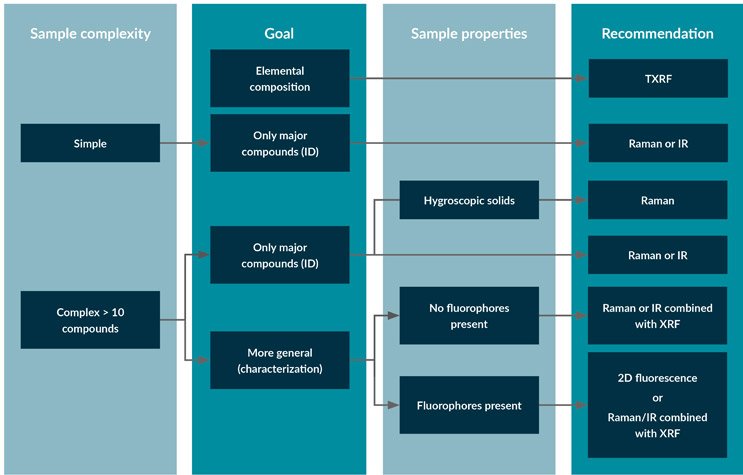
Figure 2: Decision-making chart for selecting a spectroscopic technique for fingerprinting cell culture media.
IR or Raman can be used to fingerprint simple or complex samples with low fluorophore content and where the focus lies on the major compounds. An advantage of Raman is that it can be collected through packaging and is available as handheld instrumentation for measuring powders and liquids. However, when used for powder characterization, Raman shows sensitivity to the presence of various polymorphs in the sample. Like Raman, IR can also be used for powders but will be very sensitive to the moisture content of the sample and is not recommended for this application when handling hygroscopic samples.
Total reflection X-ray fluorescence spectroscopy (TXRF) may complement IR and Raman spectroscopy. It can perform a qualitative analysis of trace metals present in raw materials that have been solubilized.
Level 3: Quantitative Testing
When more extensive fingerprinting or investigative work is required, several methods may be useful, including chromatography or inductively coupled plasma mass spectrometry (ICP-MS). Below is a brief description of some of these techniques.
Elemental Fingerprinting
ICP-MS is used to analyze individual elements in a sample by mass-to-charge ratio. The sample is usually acidified and then introduced into the instrument. An aerosol is formed and an ICP is used to form atomic ions, which are moved into the mass spectrometer, separated by mass, and then moved to the detector. The elements are identified by atomic mass, and a standard curve is used to quantify the elements of interest. The mass spectrum of a sample can be used as a fingerprint for comparison to other samples.
TXRF is an analytical tool that may be used to perform quantitative analysis of trace elements detected in solubilized raw materials. An internal standard is added to the sample and vortexed, and a small amount of the sample is pipetted onto a glass disk and dried. Once the sample analysis is complete, the concentration of each detected element is calculated based on the intensity of the fluorescence radiation using dedicated software connected to the TXRF instrument.
In addition to analyzing liquid samples, TXRF can analyze solid samples by the acid digestion of the sample. It allows for the rapid and direct qualitative and mass ratio evaluation of the elements present in the analyzed sample, with only micrograms of the sample without chemical distortion via acid digestion, displaying its micro-analytical capability.
Chromatography
Chromatography separates the components of complex samples and is used with various detectors to identify and quantify analytes that may be present at low concentrations. High- or ultra-high-performance liquid chromatography is often used in the reverse phase mode with aqueous or water-soluble samples. Chromatography columns may be reverse phase, size exclusion, ion exchange, or affinity, for example. Common detector options include ultra-violet/visible (UV/VIS), fluorescence, mass spectrometry, refractive index, conductivity, and charged aerosol. Analytes may be derivatized to enable or enhance detection. Ion chromatography can be used to analyze buffers and other process liquids.
Typically, a system with a column and detector appropriate to the analyte is chosen, the method is developed (column, mobile phase, column temperature, sample volume), and a chromatogram is generated. The peaks are measured and compared to a standard curve to quantify the analytes of interest. The retention time of the unknown sample is compared to the known reference standard to confirm the identity of the sample. For complex samples such as cell culture media, the chromatogram also provides a “fingerprint” of the sample and can be compared to other fingerprints as another method of identification.
If LC-MS is used, the mass spectrum adds another dimension to the fingerprint. Figure 3 describes how the methods from several detectors can be used for classical targeted/quantitative fingerprinting or untargeted fingerprinting using multivariate data analysis. UV/VIS is simple and inexpensive but can be subject to interferences in a complex mixture as it is not very specific.
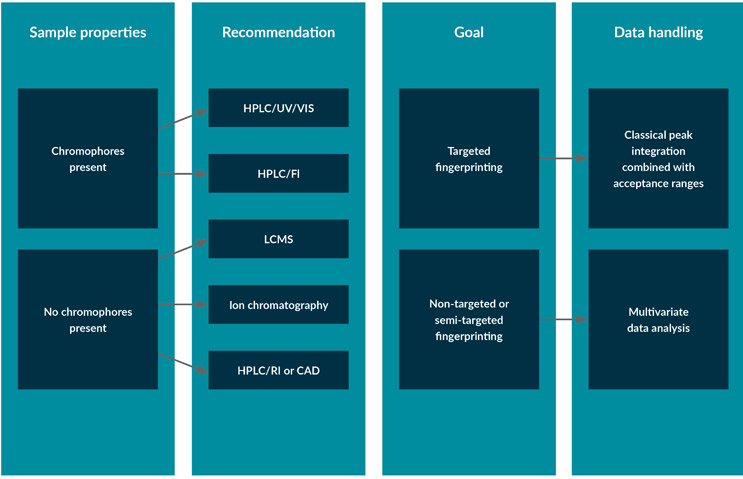
Figure 3: Decision chart for the selection of chromatographic techniques for the fingerprinting of cell culture media.
Conclusion
Although there cannot be a one-size-fits-all approach to cell culture media fingerprinting, this is an approach where “a few sizes fit most.” It allows media manufacturers/suppliers and biopharma end users to gain a deeper insight into their media and product/process capabilities and requirements and to focus their energies on what matters most in relation to materials.
This article is a summary of a recent BioPhorum publication on the topic. To read more, check out the full report in Media fingerprinting of cell culture media: a standardized analytical test method suite and three-tier approach. It has an appendix containing a comprehensive listing of common fingerprinting tools, with information to help the reader decide which tool best suits their needs.
