A closer look at gauge types
Thermal conductivity gauges
Measurement in the "rough vacuum" range below atmospheric pressure and down to about 1 millitorr (1x10-3 mm Hg) is usually done with a gauge that indirectly measures "pressure" by sensing the thermal conductivity of the remaining gas. Two types of thermal conductivity gauges are common, the thermocouple (TC) gauge and the Pirani gauge.
In the thermocouple gauge, the temperature of a very fine heated wire is sensed with an attached thermocouple. A constant electric current heats the wire. The temperature of the wire depends on the heat loss by radiation, conduction through the wire (and the thermocouple) to the connections, and transfer to the surrounding gas molecules. The sensor is carefully designed to minimize the first two effects. The temperature of the wire (as measured by the thermocouple) is at a maximum at low pressures and decreases as the gas density and gas conduction increases. The lower pressure limit of a TC gauge is about 10-3 Torr, at which point gas conductivity becomes insignificant compared to other heat loss effects. The upper end of the pressure range is around 1 Torr, depending on the gas type. For most gases the thermal conductivity is nearly constant at higher pressures as a hot gas sheath forms around the heated wire and acts as a thermal barrier. Because the gas temperature can change with rapid pressure changes, the response time for thermal sensors is slow, especially at higher pressures. A TC gauge should not be used in critical applications.
In a Pirani gauge, named after Manfred von Pirani, who first described this type of gauge in 1906, the temperature is inferred from the resistance of the wire (which depends on its temperature) and does not require a thermocouple. In a modern Pirani gauge the sensor wire is maintained at a constant resistance using a bridge circuit and feedback amplifier. Thus the wire temperature is kept constant and the bridge voltage is a measure of the temperature. Since both the TC gauge and the Pirani gauge both depend on the thermal conductivity of the gas, their low pressure limits are similar, but a constant-temperature Pirani can operate up to about 100 Torr. The response time of a Pirani gauge is also faster than a constant current TC gauge.
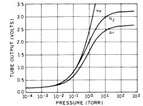
Thermal vacuum gauges are indirect pressure gauges and the output depends on the type of gas in the vacuum system. Most gauges are calibrated to read in "nitrogen-equivalent" pressure. During the pumpdown of a vacuum chamber, in the pressure ranges above 1 mTorr, the gas is mainly air and a nitrogen-calibrated gauge will read accurately. When a thermal vacuum sensor is used for vacuum processes that use other gases, such as argon, helium or hydrogen, a different calibration is required and above 1 Torr the reading relative to nitrogen diverges significantly. Figure 1 shows the relationship between actual pressure and sensor output for a Pirani gauge. For example, when the nitrogen-equivalent pressure (N2) gauge reading is1 Torr (2.0 V output), the actual helium (He) pressure will be lower and the argon (Ar) pressure will be higher.
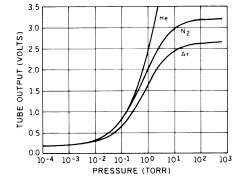
The convection Pirani gauge extends the pressure range up to and slightly higher than atmospheric pressure (Figure 2). The high-pressure sensitivity is achieved by (1) increasing the volume of the sensor enclosure (resulting in a larger sensor tube diameter); and (2) locating the sensor horizontally to promote thermal convection around the filament and disrupt the thermal sheath. In this geometry, thermal convection currents in the surrounding gas contribute to filament cooling in the pressure range above 10-100 Torr. For this reason most convection Pirani gauges must be positioned horizontally to operate properly.

Direct pressure gauges
Direct-reading pressure gauges measure actual pressure and, unlike thermal conductivity gauges, the reading is independent of gas composition. Examples of direct-reading pressure gauges include capacitance manometers, mechanical dial-type Bourdon gauges, and mercury manometers. In a capacitance manometer, such as the MKS Baratron, one side of a thin metal diaphragm is sealed at high vacuum and the other side is exposed to the pressure being measured. The displacement of the diaphragm is detected by sensing the change in capacitance between the membrane and a fixed conductive plate.
